Carbon Capture: Direct Air Capture filters CO₂ directly from the air. Adsorptive processes, such as TVSA, enable separation despite low CO₂ concentrations. Simulations help evaluate energy requirements, material selection, and process parameters to optimize DAC for efficiency and climate impact.

Created with AI
Climate change is advancing – and to mitigate its serious consequences, it is no longer enough to simply reduce emissions. According to the Intergovernmental Panel on Climate Change (IPCC), so-called negative emission technologies (NETs) are needed to remove excess carbon dioxide from the atmosphere. One of these key technologies is direct air capture (DAC), i.e., the direct removal of CO₂ from ambient air. But how exactly does this process work?

Let's start with the basics: Adsorption-based DAC processes are usually cyclical, alternating between an adsorption and a desorption phase.
Depending on how the thermodynamic equilibrium is shifted in favor of desorption, a distinction is made between different methods:
1. Lowering the gas pressure (0 → 1)
Here, desorption is forced by pressure changes. Two approaches are common:
Both methods can also be combined. The energy for desorption is provided by mechanical work.
2. Increasing the temperature (0 → 2)
In temperature swing adsorption (TSA), desorption is triggered by the application of heat. Adsorption usually takes place at ambient temperature, while desorption takes place at higher temperatures. The necessary energy is therefore supplied thermally.
3. Changing the gas composition (0 → 1)
This process relies on reducing the partial pressure of the adsorbate by introducing purge gas into the adsorber. The mode of operation is like PSA and VSA, but instead of reducing the overall pressure, the adsorbate is specifically displaced. This is referred to as composition swing adsorption (CSA).

The biggest challenge in capturing CO₂ from the air—unlike established adsorption processes such as oxygen production or gas drying—is the very low concentration of CO₂ in the atmosphere: it is only around 400 ppm.

Due to the low concentration of CO2, a large volume of air must be filtered to remove a small amount of CO2. Pressure swing adsorption (PSA) requires a great deal of mechanical work to compress this air to the desired overpressure. Therefore, PSA is energetically inefficient and unsuitable for direct air capture.
In order to test the suitability of other desorption methods, the choice of adsorption material is a decisive factor. In this blog article, we will focus on single-stage DAC processes only. Amin-functionalized adsorbents such as Lewatit are currently the most used for these processes.
Pure temperature swing adsorption (TSA) is ruled out due to the significant degradation of amine-functionalized adsorbents. Furthermore, TSA does not achieve a sufficiently high CO₂ concentration. Vacuum swing adsorption (VSA) is technically challenging because desorption only begins at pressures below 0.4mbar.
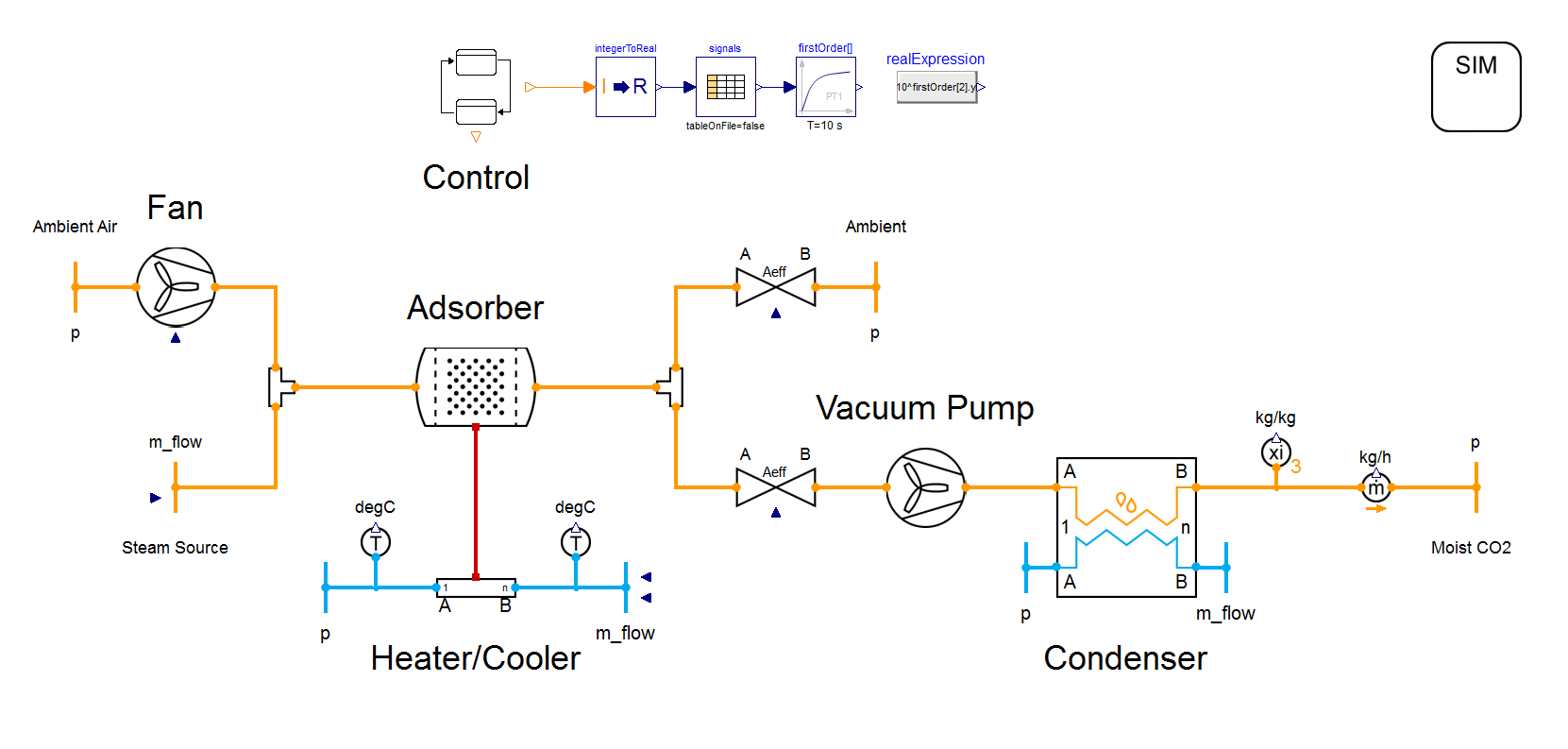
In practice, temperature-vacuum swing adsorption (TVSA) has established itself as the standard process for direct air capture. This process combines vacuum technology with an increase in temperature: the vacuum reduces the oxygen content in the adsorber, thereby minimizing unwanted oxidation processes during heating. Meanwhile, the negative pressure facilitates the release of CO₂and enables its recovery in a highly concentrated form.
To answer these questions, it is worth looking at the isothermal representation of the process. In this example, we analyze the adsorption material Lewatit VP OC 1065. The process data comes from our DAC Example with TIL adsorption at an ambient temperature of 10 °C and a relative humidity of 50 %. For desorption, the adsorber is evacuated to a vacuum pressure of 0.3 bar and then heated to 100 °C. Desorption without steam purging (1 → 2) and with steam purging (1 → 2 → 3) are shown in the following figure:

Steam purging expands TVSA with composition swing adsorption (CSA). In this process, steam flows through the adsorber and reduces the CO₂ partial pressure that rises during desorption (2 → 3).
Advantages:
Disadvantages:
Conclusion: Steam purging offers several advantages for CO₂ capture with DAC. However, it is not always energy efficient. Therefore, it is crucial to carefully analyze the energy requirements per ton of CO₂ captured to thoroughly evaluate the economic efficiency and sustainability of steam purging in the DAC process.
How simulation can help you:
Overall, the simulation of DAC processes enables targeted process optimization, reduces development costs, and provides valuable insights for scaling up to industrial scale
With TIL Adsorption and our project support, these analyses can be carried out efficiently and turned into practical results.
Our expertise in system simulation helps you identify opportunities in Direct Air Capture, overcome challenges, and apply this technology in a targeted way.
From material modeling to optimized process design, we support you with expertise and tailored solutions. Simply schedule a non-binding consultation – and discover the benefits of the specialized simulation library TIL Adsorption as well as our comprehensive engineering services.